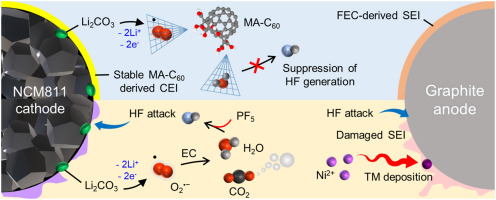
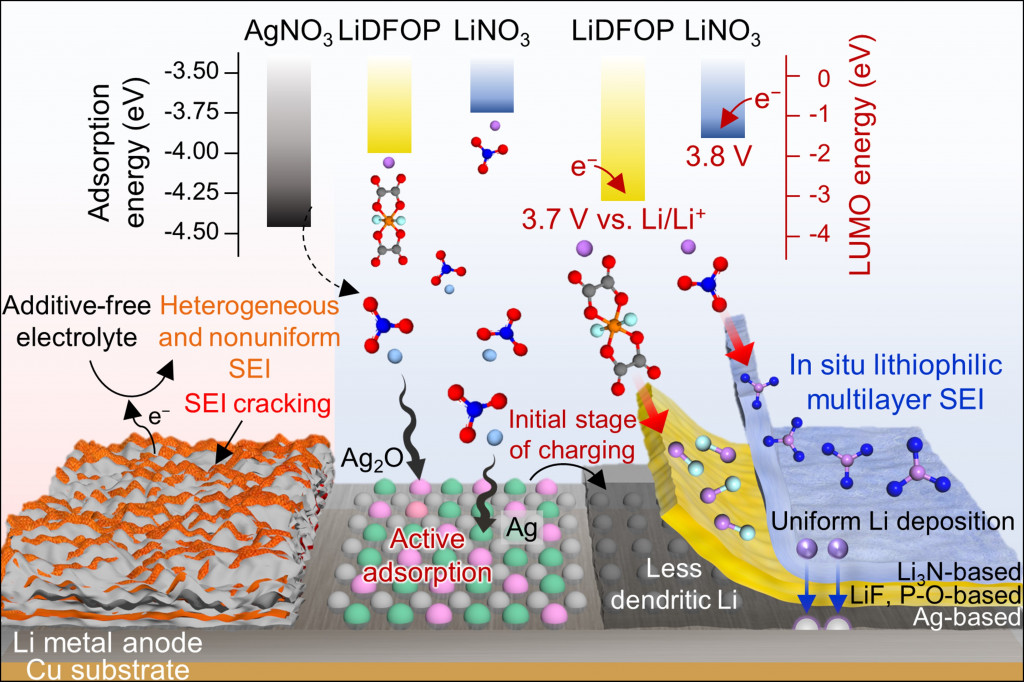
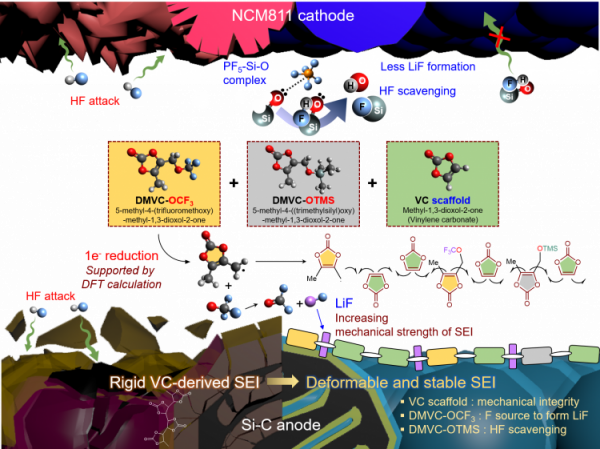
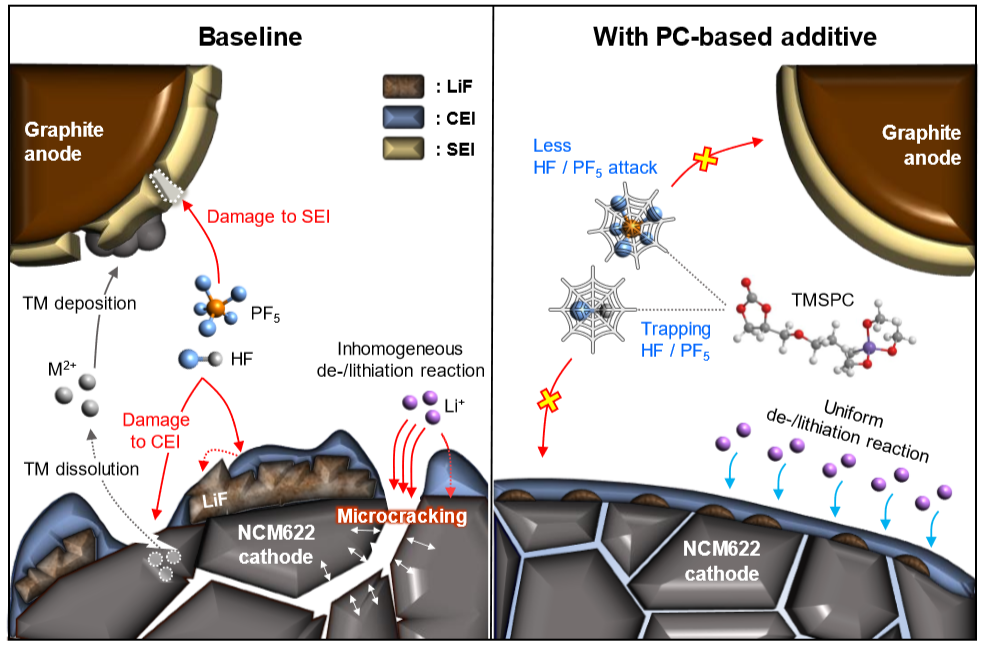
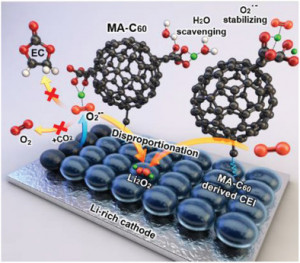
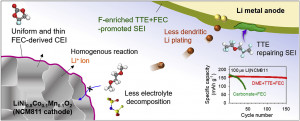
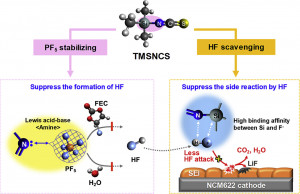
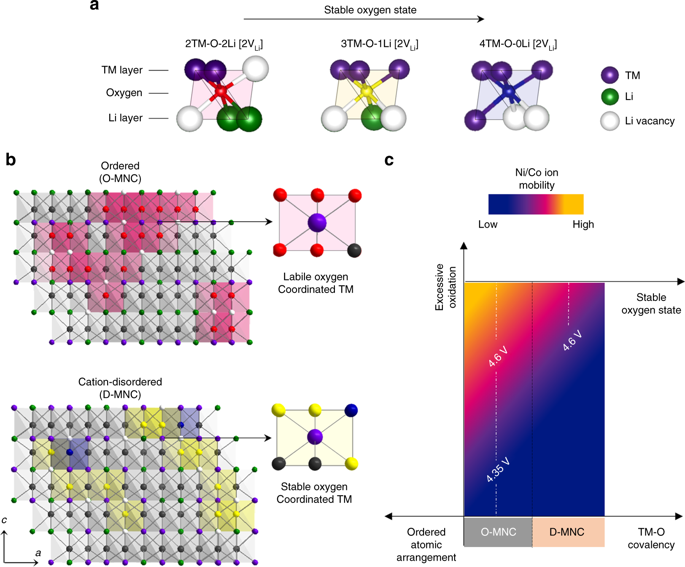
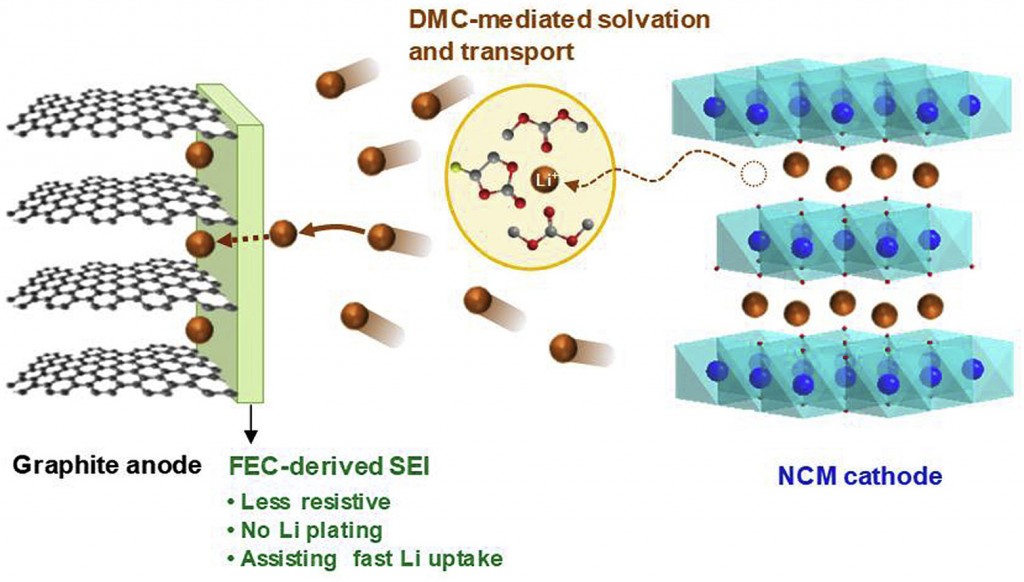
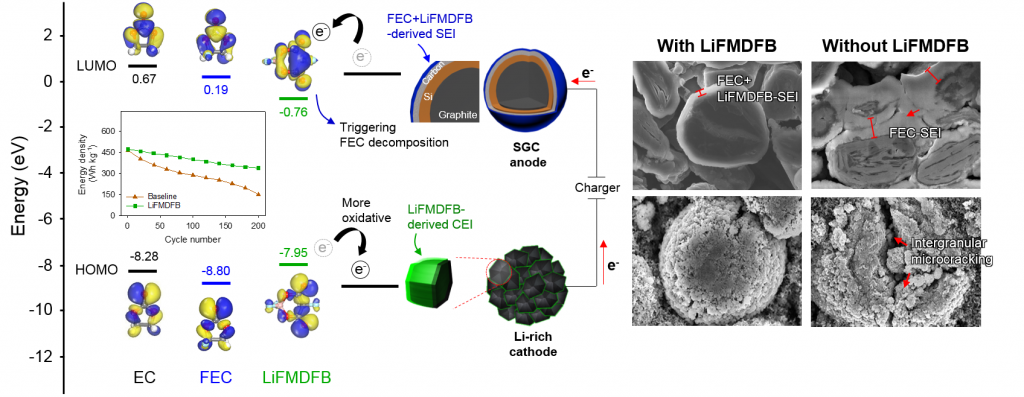
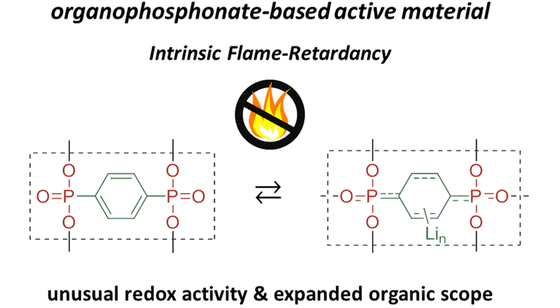
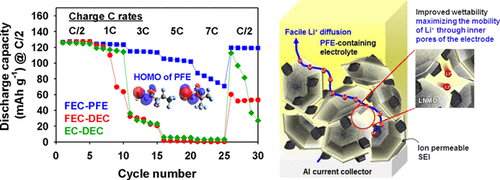
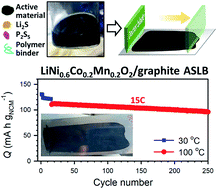
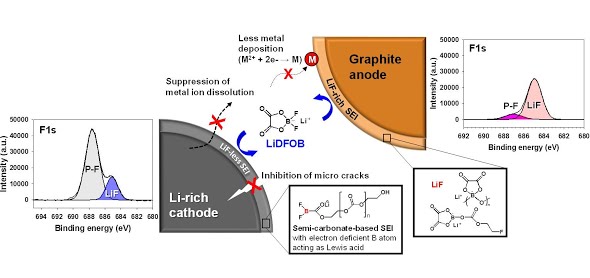
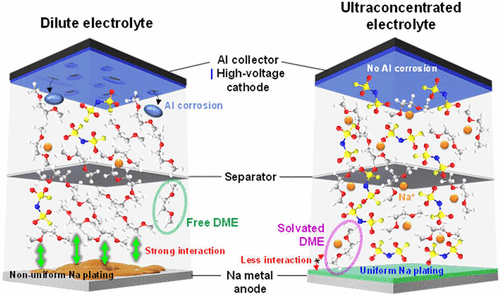
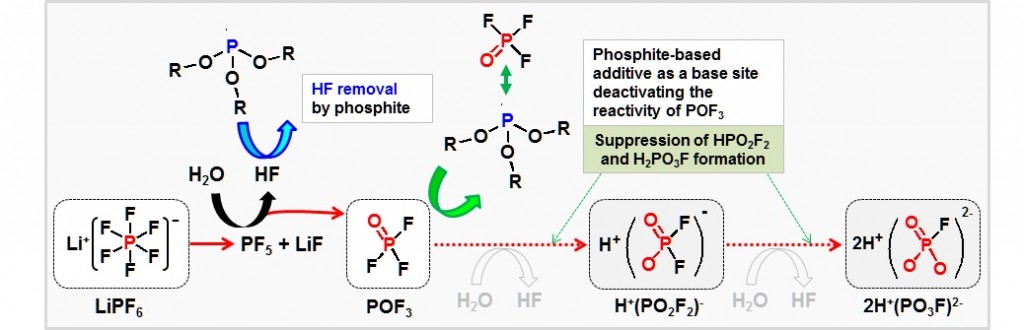
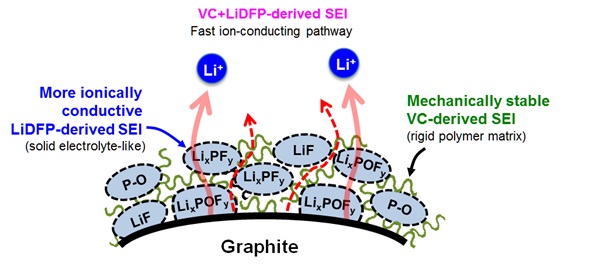
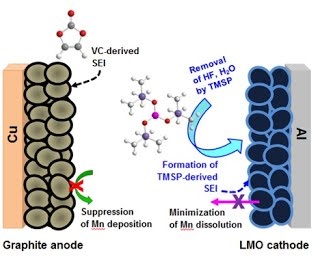
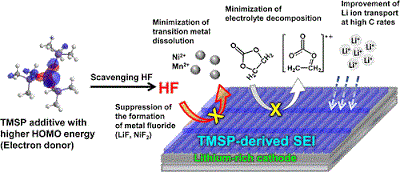
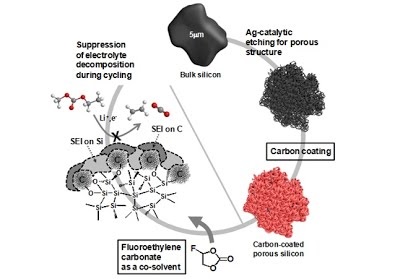
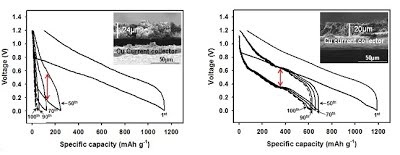
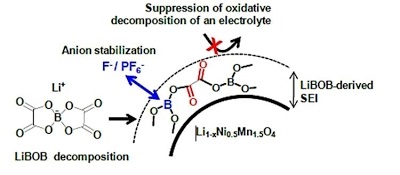
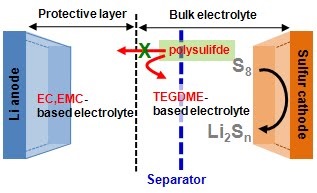
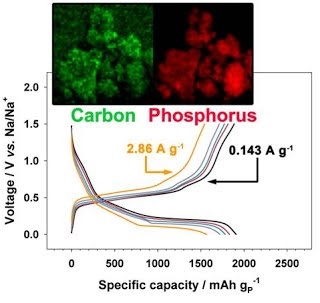
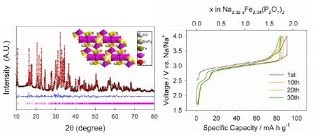
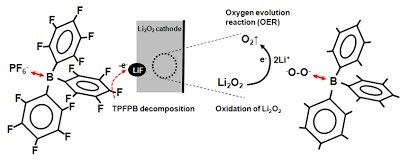
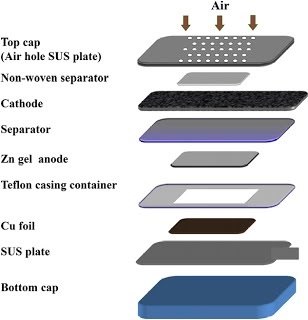
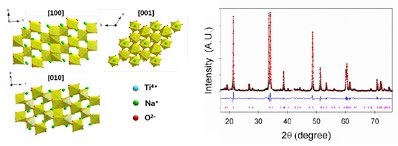
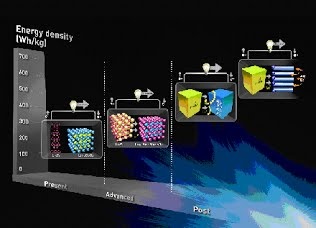
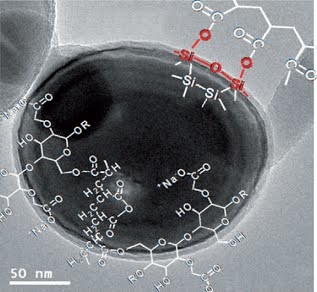
Long-term stability of the solid electrolyte interphase (SEI) and cathode-electrolyte interface (CEI) layers formed on anodes and cathodes is imperative to mitigate the interfacial degradation of electrodes and enhance the cycle life of lithium-ion batteries (LIBs). However, the SEI on the anode and CEI on the cathode are vulnerable to the reactive species of PF5 and HF produced by the decomposition and hydrolysis of the conventional LiPF6 electrolyte in a battery inevitably containing a trace amount of water. Here, we report a new class of cyclic carbonate-based electrolyte additives to preserve the integrity of the SEI and CEI in LIBs. This new class of additives is designed and synthesized by an ecofriendly approach which involves fixing CO2 with functional epoxides bearing various reactive side chains. It was found that the cyclic carbonates of 3-(1-ethoxyethoxy)-1,2-propylene carbonate (EEPC) and 3-trimethoxysilyl propyloxy-1,2-propylene carbonate (TMSPC), possessing high capability for the stabilization of Lewis-acidic PF5, exhibit a capacity retention of 79.0% after 1000 cycles, which is superior to that of the pristine electrolyte of 54.7%. Moreover, TMSPC has HF scavenging capability, which, along with PF5 stabilization, results in enhanced rate capability of commercial LiNi0.6Mn0.2Co0.2O2 (NCM622)/graphite full cells, posing a significant potential for high-energy density LIBs with long cycle stability.
- External link of publication
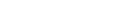










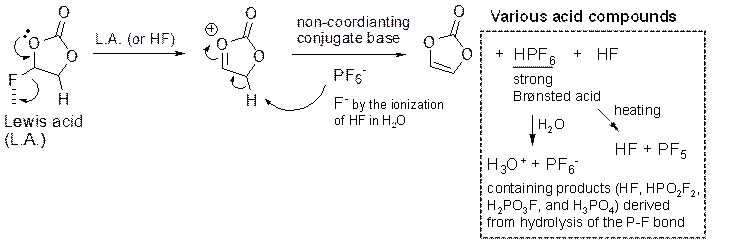






 O and C
O and C O
O