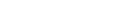< Our group’s outstanding works: electrolyte additives, electrolyte formulation, binders >
- FEC : Fluoroethylene carbonate
- Crosslinked binder : Crosslinked structure based on poly(acrylic acid) and carboxymethyl cellulose
- TMSPi : Tris(trimethyl)silyl phosphite
- Mg(TFSI)2-diglyme : Magnesium (II) bis(trifluoromethane sulfonyl)imide dissolved in a mixed solvent of dimethoxyethane and diethyleneglycol dimethyl ether
Our group focuses on the mechanistic analysis of electrode-electrolyte interfaces and the development of tailored electrolyte systems that can ensure good battery performance. We propose unique and effective solutions to improve the interfacial stability of high-voltage and high-capacity electrodes without degradation of battery performance.
Our pioneer research sparked the study of electrolytes and polymeric binders for further improvement of battery performance. Furthermore, we are developing a new electrolyte formulation using our high-level electrolyte technology to produce long-lasting alkali (Li, Na, and Mg) metal batteries and realize the highly safe operation of batteries.
■ Molecularly engineered electrolyte additives for stabilizing the high-voltage cathode-electrolyte interface
Attempts to achieve long cycle life in batteries with higher energy density have been intensively undertaken for practical use in applications such as electric transportation and large-scale energy grid systems. The interfacial engineering of electrode materials using electrolyte additives offers great promise for high-performance batteries. High-voltage cathode materials have recently gained attention as promising candidates for improving the energy density of lithium-ion batteries (LIBs). Reliable performance of LIBs will be ultimately achieved through the advancement of structurally optimized electrode materials coupled with tailored electrolyte systems.
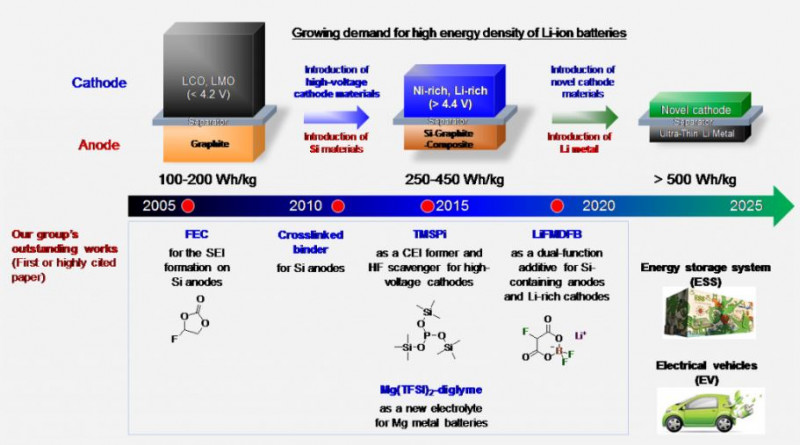
■ Functional electrolyte additives for the construction of cathode–electrolyte interface (CEI)
The use of advanced electrolyte additives with unique functions such as the ability to form a stable artificial protective layer on the anode and the cathode in situ has recently been recognized as a promising means of obtaining highly reliable LIBs with high energy density. The formation of a controlled CEI by electrolyte additives is a promising strategy to enhance the electrochemical performance of high-voltage cathodes while alleviating the continued occurrence of unfavorable side reactions. Recent advancements in the development of scavengers with high selectivity and affinity toward unwanted species indicate that the use of these additives can effectively mitigate the problem of electrolyte instability that degrades battery performance and reduces the lifetime [Energy & Environmental Science (2018), ChemElectroChem (2016), ACS Applied Materials & Interfaces (2015), Journal of Materials Chemistry A (2014)].
< Effective approaches for the stabilization of high-voltage cathode-electrolyte interface >
■ Reductive electrolyte additives for the protection of high-capacity anodes
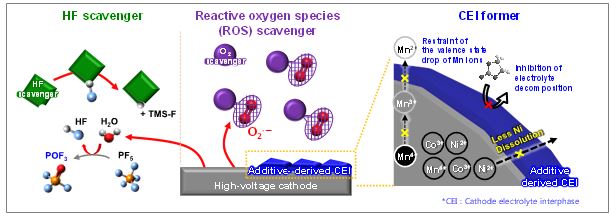
As the demand for high-energy-density LIBs is rapidly increasing, the development of high-capacity electrode materials is imperative. In particular, Si is a promising anode material because of its high theoretical capacity of 3579 mAh g-1 for Li15Si4, surpassing that of the carbonaceous anodes (372 mAh g-1 for LiC6) present in current commercial products. The development of electrolyte additives is focused on the formation of a robust and stable artificial solid-electrolyte interphase (SEI) on the anode. Because protection by the SEI against unwanted anode–electrolyte reactions affects the electrochemical properties of the anodes, the nature of the SEI on the anode is critically important. To properly design electrolyte additives that form anode SEIs, their lowest unoccupied molecular orbital (LUMO) energy is generally compared with that of EC as a conventional solvent. The most frequently used reducible additives in Li-ion cells with graphite and Si anodes are vinylene carbonate (VC) and fluoroethylene carbonate (FEC) that have relatively low LUMO energy levels compared to EC. Very recently, our group developed a new electrolyte additive containing fluoro(malonato)difluoro borate (FMDFB) anion that has a relatively low LUMO energy level compared to FEC, reflecting a high electron affinity. This makes it probable that the FMDFB may be reduced prior to the FEC decomposition at the anode.
< Diagram of the HOMO and LUMO energy levels of non-ionic and ionic additives >
- EC : Ethylene carbonate
- FEC : Fluoroethylene carbonate
- VC : Vinylene carbonate
- LiDFOB : Lithium difluoro(oxalato)borate
- LiFMDFB : Lithium fluoro(malonato)difluoro borate
■ Development of new reductive additives and acid stabilizers (scavengers) in electrolytes
Our group has continued to develop new additives for stable SEI formation and developed a complementary electrolyte design that enables interfacial structuring through combination with conventional electrolyte additives. Our recent results demonstrate that the dual-segment SEI formation conserving the electrochemical activity of the Si-embedded anode will assist the development of a new class of electrolyte additives for high-capacity anodes [Energy & Environmental Science (2018)]. Very recently, we have developed reductive additives that have the ability to construct a mechanically and electrochemically stable SEI on Si-containing anodes, and we plan to design several possible derivatives of these additives through molecular tuning.
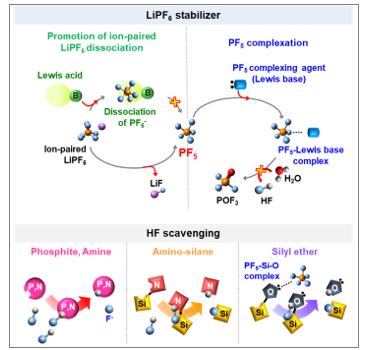
Two strategies have been proposed to stabilize LiPF6-based electrolytes. First is the deactivation of strong Lewis acids such as PF5. The formation of PF5 is reduced by the promotion of ion-paired LiPF6 dissociation (Li+PF6-) that hinders the forward reaction of the equilibrium decomposition reaction of LiPF6 (i.e., LiPF6 → LiF + PF5), or PF5 is stabilized by the formation of a complex with Lewis base additives, prohibiting further decomposition reactions of PF5 in the electrolyte. Second is scavenging of reactive species such as HF. Brønsted acidic HF can be scavenged by Lewis base additives with various functional moieties. Our group demonstrated that the scavenging of HF in electrolytes is mainly achieved by its complexation with basic electron-donating sites such as nitrogen cores, silanes, and phosphites [ChemElectroChem (2016), Journal of Materials Chemistry A (2014)].
< Strategies for stabilization of LiPF6-based electrolytes>